A team from MIT describes this new tool, PASTE DNA editing that delivers genes as long as 36,000 DNA base pairs to several types of cells. The research is published in Nature Biotechnology.
What is PASTE DNA editing?
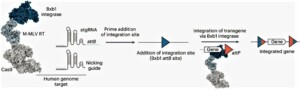
PASTE is short for “programmable addition via site-specific targeting elements”. PASTE uses a CRISPR–Cas9 nickase fused to two enzymes – a reverse transcriptase and a serine integrase – for targeted genomic recruitment and integration of DNA. It achieves the integration of large pieces of DNA without double-stranded DNA breaks. It aims at integrating whole genes, not just correcting individual mutations.
How is PASTE DNA editing related to CRISPR editing tool?
Both PASTE and CRISPR use the ongoing battle between bacteria and the viruses that infect them. For this study, the researchers focused on serine integrases, which integrate the large viral genome in bacterial DNA. These enzymes target specific genome sequences known as attachment sites. When they find the site in the host genome, they bind to it and integrate their DNA payload. The MIT team combined these enzymes with a CRISPR-Cas9 system that recognizes the new targeted sequence for insertion of the DNA payload.
PASTE includes a Cas9 enzyme that cuts at a specific genomic site, guided by a strand of RNA that binds to that site. This allows them to target any site in the genome for insertion of the attachment site, which contains 46 DNA base pairs. This insertion can be done without introducing any double-stranded breaks by adding one DNA strand first via a fused reverse transcriptase, then its complementary strand. Once the site is incorporated, the integrase can come along and insert its much larger DNA payload into the genome at that site.
In this study, the researchers showed that they could use PASTE to insert genes into several types of human cells, including liver cells, T cells, and lymphoblasts. They tested the delivery system with 13 different payload genes, including some that could be therapeutically useful, and were able to insert them into nine different locations in the genome.
The authors noted that PASTE has editing efficiencies “similar to or exceeding those of homology-directed repair and non-homologous end joining-based methods, with activity in nondividing cells and in vivo with fewer detectable off-target events.”
The researchers also demonstrated that they could insert genes in “humanized” livers in mice. Livers in these mice consist of about 70% human hepatocytes, and PASTE successfully integrated new genes into about 2.5% of these cells.
Prospects of PASTE DNA editing
The researchers are now further exploring the possibility of using PASTE DNA editing as a possible way to replace the defective cystic fibrosis gene. This technique could also be useful for treating blood diseases caused by faulty genes, such as hemophilia and G6PD deficiency, or Huntington’s disease, a neurological disorder caused by a defective gene that has expanded triplet repeats.
Sources:
PASTE Expands CRISPR Toolbox by Inserting Large Pieces of DNA.
See also:
Go to the News Board