Cancer is a complex and heterogeneous disease that affects millions of people worldwide. Despite the advances in diagnosis and treatment, many patients still face poor outcomes and high mortality rates. One of the challenges in cancer research is to understand the molecular mechanisms that drive cancer initiation, progression and resistance to therapy. This is where cancer omics comes in.
What is cancer omics?
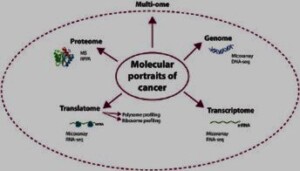
Cancer omics is a branch of science that uses high-throughput technologies to analyze the genomic, transcriptomic, proteomic and metabolomic profiles of cancer cells and tissues. These technologies generate large amounts of data that can reveal the genetic and epigenetic alterations, gene expression patterns, protein interactions and metabolic pathways that characterize different types of cancer. By integrating and interpreting these data, cancer omics can provide insights into the molecular signatures, subtypes, biomarkers and therapeutic targets of cancer.
How does cancer develop?
What exactly is cancer, and how does it develop? Is it a genetic disease, caused by mutations in our DNA, or a metabolic disease, caused by disruptions in our cellular energy production? This question has been debated for decades, and the answer may have important implications for how we prevent and treat cancer.
The Genetic Theory of Cancer
The genetic theory of cancer is the dominant view in the field of oncology. It states that cancer is a genetic disease, meaning that it is caused by changes in genes that control the way our cells function, especially how they grow and divide. Genes are sections of DNA that carry instructions to make proteins, which are essential for various cellular processes. Sometimes, genes can become damaged or altered by random errors, environmental factors, or inherited mutations.
Some mutations can affect genes that regulate cell growth and division, such as oncogenes (which promote cell growth) and tumor suppressor genes (which inhibit cell growth). When these genes are mutated, they can cause cells to grow and divide uncontrollably, forming tumors. Cancer cells can also acquire mutations that allow them to evade the immune system, invade other tissues, and spread to other parts of the body (metastasis).
According to the genetic theory of cancer, each cancer is unique and has its own set of mutations that drive its development and progression. Scientists have identified hundreds of genes that can be mutated in different types of cancers. For example, mutations in the BRCA1 or BRCA2 genes can increase the risk of developing breast and ovarian cancers. However, inheriting a cancer-related mutation does not mean that one will definitely develop cancer; it only means that the risk is increased. Most cancers are not inherited, but occur by chance as a result of accumulating mutations over time.
The Metabolic Theory of Cancer
The metabolic theory of cancer is an alternative view that challenges the genetic theory. It states that cancer is a metabolic disease, meaning that it is caused by disruptions in the normal metabolism of cells. Metabolism is the process by which cells convert nutrients into energy and building blocks for various cellular functions. The main source of energy for most cells is glucose, which is broken down through a series of chemical reactions in the cytoplasm (glycolysis) and in the mitochondria (oxidative phosphorylation).
The metabolic theory of cancer proposes that cancer cells have defective mitochondria, which are the organelles responsible for oxidative phosphorylation and energy production. As a result, cancer cells rely mainly on glycolysis for energy, even in the presence of oxygen (a phenomenon known as the Warburg effect). This allows them to survive and proliferate in low-oxygen environments, such as solid tumors. Cancer cells also have altered metabolism of other nutrients, such as amino acids and fatty acids, which enable them to synthesize the building blocks they need for rapid growth and division.
According to the metabolic theory of cancer, mitochondrial defects are the primary cause of cancer, while genetic mutations are secondary consequences that arise from metabolic stress . The metabolic theory suggests that cancer is not as unique or diverse as the genetic theory implies, but rather has common features that can be targeted by metabolic interventions. For example, restricting glucose intake or inhibiting glycolysis may starve cancer cells of their preferred fuel source and slow down their growth.
The Multi-Omics Perspective
While both the genetic theory and the metabolic theory of cancer have their merits and limitations, they are not mutually exclusive. In fact, they may be complementary and interconnected. The genome (the set of genetic instructions), the exposome (the set of environmental exposures), and the metabolome (the set of metabolic products) all interact with each other in a complex feedback loop that influences the development and progression of cancer. For example, genetic variants can affect the expression and activity of enzymes that regulate energy metabolism, and metabolic stress can induce DNA damage and genetic instability. Environmental factors can also modulate both genetic and metabolic pathways.
Therefore, a more comprehensive and holistic perspective that considers all these factors in unison may be more useful for understanding and addressing cancer. This perspective is called the multi-omics approach, which integrates data from different levels of biological information (such as genomics, exposomics, metabolomics, proteomics, etc.) to reveal the complex interactions and mechanisms that underlie cancer. The multi-omics approach may help identify new biomarkers, targets, and strategies for cancer prevention and treatment that are more personalized and effective.
Applications of cancer omics
Cancer omics has several applications in cancer research and clinical practice. For example, cancer omics can help to identify novel genes and pathways involved in cancer development and progression.
By integrating and analyzing the data, cancer omics can provide insights into the molecular signatures, pathways and networks that are dysregulated in cancer and how they interact with each other and with the tumor microenvironment. These insights can help identify novel biomarkers for diagnosis, prognosis and prediction of response to therapy, as well as new targets for drug development and personalized medicine.
Cancer omics also has the potential to improve the classification and stratification of cancer patients based on their molecular profiles, rather than relying on traditional histological or clinical criteria. This can enable a more precise and tailored approach to cancer treatment that takes into account the molecular heterogeneity and diversity of cancer and its evolution over time.
Cancer omics can also help to monitor tumor evolution and response to therapy over time. Proper analysis of data can identify mechanisms of resistance to therapy and new strategies to overcome them.
Challenges to cancer omics
Cancer omics is a promising field that has the potential to improve our understanding and management of cancer. However, it also faces some challenges and limitations, such as the complexity and heterogeneity of cancer, which makes it difficult to capture the full spectrum of molecular changes and interactions in a single omics platform.
Another challenge is the need for standardized methods and protocols for data generation, analysis and interpretation across different laboratories and studies. There are also the ethical and legal issues related to data sharing, privacy and consent of patients and donors. The translation of omics findings into clinical practice requires validation in larger cohorts, clinical trials and real-world settings.
In summary
Cancer omics is an exciting and rapidly evolving field that offers new opportunities and challenges for cancer research and care. By collaborating across disciplines and sectors, we can harness the power of omics data to better understand, prevent, and treat cancer.
See also:
All You Need To Know About Multiomics Use