Cancer blood tests? This is a rather old news. What brings it up is the ongoing discussion here about liquid biopsy. In fact, the term “blood test” is now giving way to the term “liquid biopsy” in the field of oncology .
Doctors have long made their treatment decisions for cancer based on features like the organ in which the cancer started growing and whether the cancer has metastasized. Now, they often base their decisions on the genetic changes in the tumor.
Cancer blood tests, liquid biopsies, by analyzing the circulating tumor-derived DNA provide genomic profiling of the tumor. Oncologists can use this tumor profiling to direct their targeted therapy.
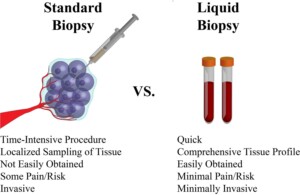
Liquid biopsies or cancer blood tests have the advantage of being minimally invasive. In certain cases, it is difficult to obtain a tissue biopsy, or the needle biopsy may not be well representing the whole tumor. Here comes the beneficial role of cancer blood tests or tissue biopsies.
The Food and Drug Administration (FDA) has approved, in August 2020, two cancer blood tests that can help guide treatment decisions. The tests, Guardant360 CDx and FoundationOne Liquid CDx, are made by different companies, Guardant Health and Foundation Medicine, and were approved separately.
GRAIL, a healthcare company whose mission is to detect cancer early, when it can be cured, introduced a multi-cancer early detection test named Galleri. This new cancer blood test can detect more than 50 types of cancer through a single blood draw. Forty-five of those cancer types don’t currently have another recommended screening. Statistics show that over 70% of cancer deaths are caused by cancers not commonly screened for.
The earlier that cancer can be found, the higher the chance of successful treatment and survival. Galleri is a revolution in this area, according to GRAIL. By adding Galleri to existing screening tests, there is the potential to reduce late-stage cancer diagnoses leading to reductions in the human and economic toll of cancer.
Such a cancer blood test could only be produced using the power of next-generation sequencing, population-scale clinical studies, and state-of-the-art computer science and data science. Liquid biopsies haven’t yet replaced a tumor biopsy. But liquid biopsies are showing great promise in facilitating individualized approaches to cancer treatment.
What about the drawbacks? Are there any? Overdiagnosis is one of these though false positive results are claimed to be less than 1%. Some slow cancers may not show at all, letting the patient to live a completely cancer-free life. Some cancers are still treatable even if diagnosed at a late stage. Early discovery of cancer in these situations will surely be a burden, at least to the patient.
What about false negative results? Is sensitivity 100%? Theoretically, a tumor may not shed enough DNA to the blood to be detected and profiled. A negative result should then be interpreted as inconclusive.
One important issue remains, which is the high cost of this test. It is approximately 1000 US dollars. Hopefully, this cost may go down in the future, or the insurance and health agencies may find it cheaper than advanced cancer treatment.
Will these great expectations for the cancer blood tests come true? This question is going to be answered in the near future.
Update: Cancer Blood Test Shows Low Positive Predictive Value
Go to the News Board
That is great.
Now one can go to the lab, give them a blood sample and say:
“Check me out, see if I have a cancer”.
Pingback: Liquid Biopsy Automation - Bioinformatics Hub
Pingback: Aristotle Multi-Cancer Test - Bioinformatics Hub
Pingback: Early Cancer Detection by Metabolism-Based Test - Bioinformatics Hub