Gene editing for Sickle Cell Disease treatment succeeded. This is the story told by BBC about Jimi, one of the study subjects of this treatment trial. According to BBC, this treatment gave him a new life instead of the pain-filled life he had before.
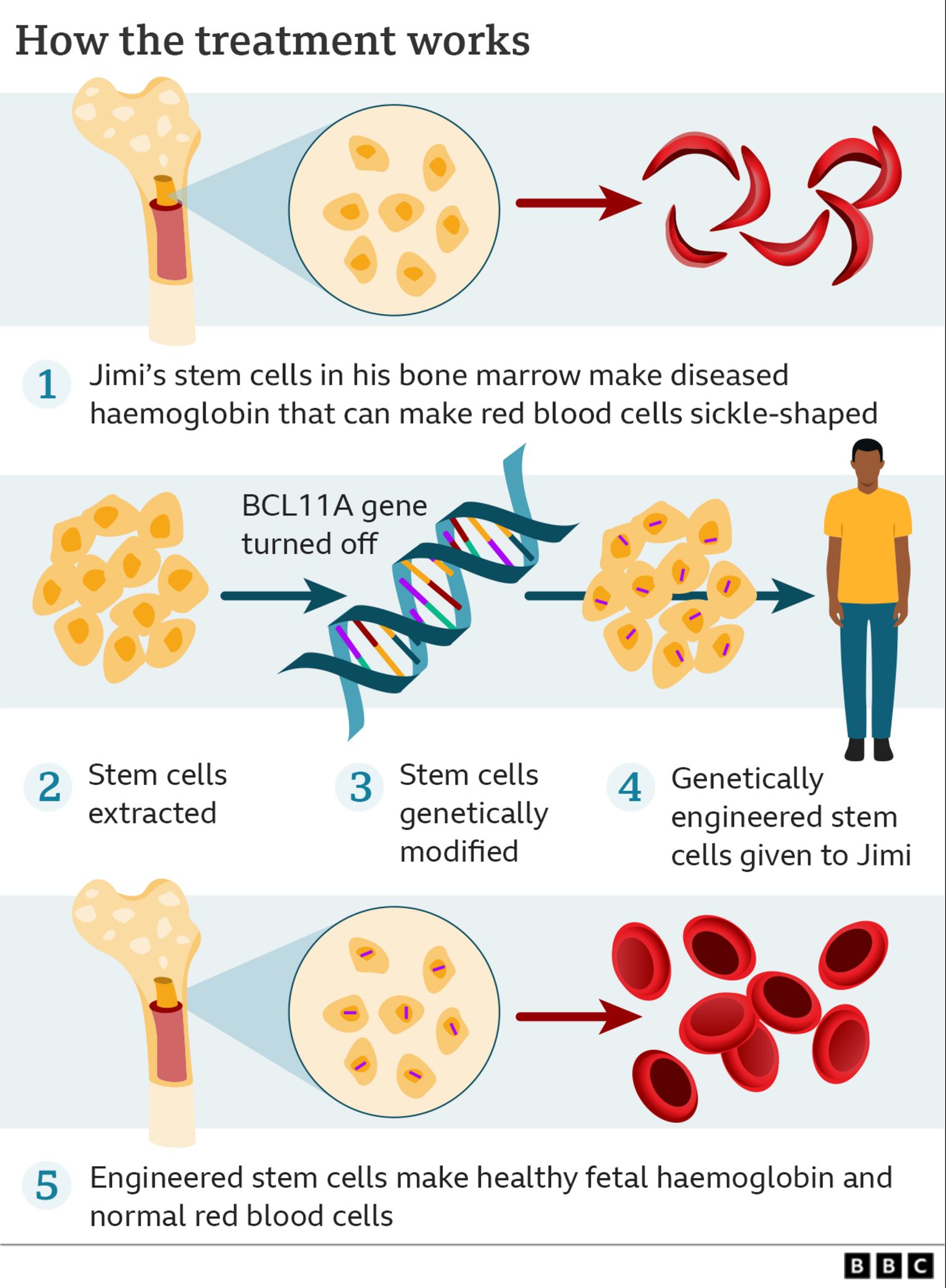
The procedure as described involved the collection of autologous bone marrow stem cells, modifying these cells ex-vivo, then reinjecting them in the patient after killing his disease-producing hematopoietic stem cells by chemotherapy.
Sickle Cell Disease results from a point mutation of the hemoglobin beta-chain gene. This makes hemoglobin sticky, which makes the red blood cells to sickle. Abnormally shaped cells get stuck in the narrow blood vessels. This causes occlusion of the blood vessels and the painful sickle cell crises.
Normal adult hemoglobin, hemoglobin A1, contains two alpha-chains and two beta-chains. It replaces fetal hemoglobin shortly after birth. Fetal hemoglobin, hemoglobin F, contains two alpha-chains and two gamma-chains. By switching from the production of hemoglobin F to hemoglobin A1, the sickle cell patient starts to suffer.
The idea of the gene-editing treatment Jimi had was to switch him back to the production of fetal hemoglobin, which does not cause sickling of cells. This means to turn off the switch that caused the transition of hemoglobin production in the first place.
The mechanism of switching hemoglobin production from F to A1 involves the gene known as BCL11A. Knocking down this gene is the strategy for switching back hemoglobin F production for treatment of sickle cell disease, like in Jimi’s case. CRISPR/Cas9 gene-editing technique was used for this purpose.
“Our approach is to turn that switch off and increase the production of fetal hemoglobin again, basically turning the clock back,” says Dr. Haydar Frangoul, who treated Jimi at the Sarah Cannon Research Institute. You can listen to Dr. Haydar Frangoul describing this approach on Youtube.
What is Crisper? Go to discussion forum
Go to the News Board