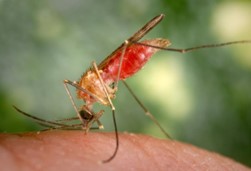
Scientists led by researchers from the Transmission:Zero team at Imperial College London have engineered genetically modified mosquitoes that slow the growth of malaria-causing parasites in their guts, and prevent transmission of the disease to humans.
Malaria remains one of the world’s most devastating diseases, putting at risk about half of the world’s population. In 2021 alone, the disease infected 241 million and killed 627,000 people, mostly children aged below five years, in sub-Saharan Africa.
The genetically modified mosquitoes carry a genetic modification that causes them to produce compounds in the gut that stunt the growth of the malaria parasites, meaning that the parasites are unlikely to reach the mosquitoes’ salivary glands and be passed on to a human in a bite before the insects die.
Production of the genetically modified mosquitoes
The Transmission:Zero team genetically modified Anopheles gambiae, the primary malaria-carrying vector in sub-Saharan Africa. Insertion of the genes of two antimicrobial peptides (AMPs) was coupled with the existing gene drive technology, aiming to promote the spread of the modification, and drastically cut malaria transmission. CRISPR gene drive is a genetic trick that causes preferential inheritance of the antiparasite genetic modification, making it spread more widely among any natural population.
On taking a blood meal, the transgenic mosquitoes produce two antimicrobial peptides in the gut, which impair the malaria parasite’s development. The two peptides are magainin 2, found within skin secretions of the African claw frog Xenopus laevis, and melittin, a primary toxin component of the European honeybee Apis mellifera. Inhibiting the parasite’s development causes a few days’ delay before the next parasite stage could reach the mosquito salivary glands, by which time most mosquitoes in nature are expected to die.
The peptides work by interfering with mitochondrial function and the energy metabolism of the parasite. They also have some effect on the mosquitoes, causing them to have a shorter lifespan and further decreasing their ability to pass on the parasite. As the modification additionally reduces female mosquito life span, the possibility of infectious sporozoites to be transmitted to a new host is reduced markedly.
Next steps with the genetically modified mosquitoes
The research team showed that their strategy can dramatically reduce the possibility of malaria spreading, in a lab setting. If the genetic modification is to help prevent malaria spread in the real world, it will need to be spread from lab-bred mosquitoes to wild populations. If all goes well, field trials are anticipated within 2–3 years. If proven safe and effective in real-world settings it could offer a powerful new tool to help eliminate malaria.
With partners in Tanzania, the team has set up a facility to generate and handle genetically modified mosquitoes and conduct some initial tests. These include collecting parasites from locally infected schoolchildren, to ensure that the modification works against the parasites circulating in relevant communities.
Source:
Genetically Modified Mosquitoes Stunt Malaria Parasite Growth, Prevent Transmission (genengnews.com)
Hoermann A. et al. Gene drive mosquitoes can aid malaria elimination by retarding Plasmodium sporogonic development. Science Advances 21 Sep 2022 Vol 8, Issue 38 DOI: 10.1126/sciadv.abo1733
See also:
Mosquito Gene Alteration to Combat Disease
Go to the News Board
Pingback: Mosquito Gene Alteration to Combat Disease - Bioinformatics Hub
The fitness cost of this procedure makes the mosquito of a shorter life span than the wild type. When released in the wild, these mosquitoes will be eliminated by natural selection.