Written by A. Hammouda: A recent article in Nature Neuroscience asserted the success of RNA-targeting CRISPR-Cas13d system in alleviating disease-related phenotypes in Huntington’s disease models.
Huntington’s disease
Huntington’s disease (HD) is a fatal, dominantly inherited neurodegenerative disorder caused by CAG trinucleotide short tandem repeat (STR) expansion in exon 1 of the Huntingtin (HTT) gene. This trinucleotide sequence codes for the amino acid glutamine, placing HD in a broader class of neurological disorders known as polyglutamine diseases. Motor symptoms can manifest from childhood to old age, with onset inversely correlated with CAG repeat length in mutant HTT, with the longer the CAG repeat, the earlier symptoms arise. The therapies currently available to patients with HD offer only moderate symptomatic relief and affected individuals typically die 15–20 years post-diagnosis due to complications.
RNA-targeting CRISPR-Cas13d system
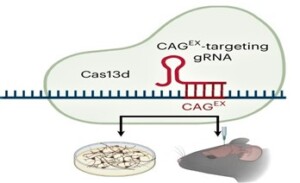
Since the normal HTT allele plays important roles during brain development as well as in adult central nervous system function, it is important to find a method for inhibiting only the mutant allele. In this study, the authors tested a CRISPR-Cas13d-based gene therapy approach that silences mutant toxic CAG-expanded (CAGEX) HTT RNA in both human and mouse models of HD. Cas13d, with a size of only approximately 930 amino acids, can be packaged and delivered with its single-guide RNA (sgRNA) to target cells via a single adeno-associated virus (AAV) capsid.
This RNA-targeting CRISPR-Cas13d system selectively reduced CAGEX HTT RNA in neurons derived from patients with HD with CAG expansions ranging from 66 to 109 STRs. AAV-mediated delivery of Cas13d–CAGEX to the striatum of disease model mice resulted in allele-selective suppression of mutant HTT mRNA and protein aggregates, while maintaining normal HTT mRNA and protein levels, significantly improved motor function and attenuated striatal atrophy.
Implications of the study
This study gives evidence that RNA-targeting CRISPR-Cas13d system can eliminate the mutant mRNA and protein, which is a promising allele-selective therapeutic strategy. Unlike gene therapies engineered from DNA-targeting CRISPR systems, which can cause irreversible nonspecific and unintended genetic modifications in the patient’s genome, targeting RNA appears a safer, viable alternative. This study succeeded in selectively silencing the mutant allele, unlike RNA interference (RNAi) and antisense oligonucleotide (ASO) strategies that do not precisely differentiate mutant HTT from the normal allele.
Source:
Morelli, K.H., Wu, Q., Gosztyla, M.L. et al. An RNA-targeting CRISPR–Cas13d system alleviates disease-related phenotypes in Huntington’s disease models. Nat Neurosci (2022). https://doi.org/10.1038/s41593-022-01207-1
See also:
Go to the News Board